Updated August 2025
The Denka Group has established quality policies in line with its management plan Mission 2030 and is conducting quality assurance activities for a wide range of products from organic chemistry using synthetic resins and resin processing, inorganic chemistry using high-performance powder control technologies, to the bio field with technologies for culturing bacteria and viruses and producing antibodies and antigens.
The Denka Group sincerely accepts the full extent of inappropriate quality conduct that has come to light, as well as the points of indication and suggestions from the external investigation committee, and has established the four "Implementation of Measures" items below to address these issues. The Denka Group will continue to put forth its utmost efforts as a group to address these issues, strengthen compliance, prevent recurrences, and restore trust.
The Company has established a Quality Assurance Department at its head office, business divisions, and factories. By promoting organic collaboration among these three layers of Quality Assurance Departments, the Company is working to improve overall quality levels across the Denka Group.
The quality risks of each product are changing due to the ongoing changes in quality requirements and laws and regulations. As part of our response to these risks, we review our quality risk assessment from market and business environment perspectives every year, and implement initiatives to reduce quality risks in a planned manner according to the nature and magnitude of the risks.
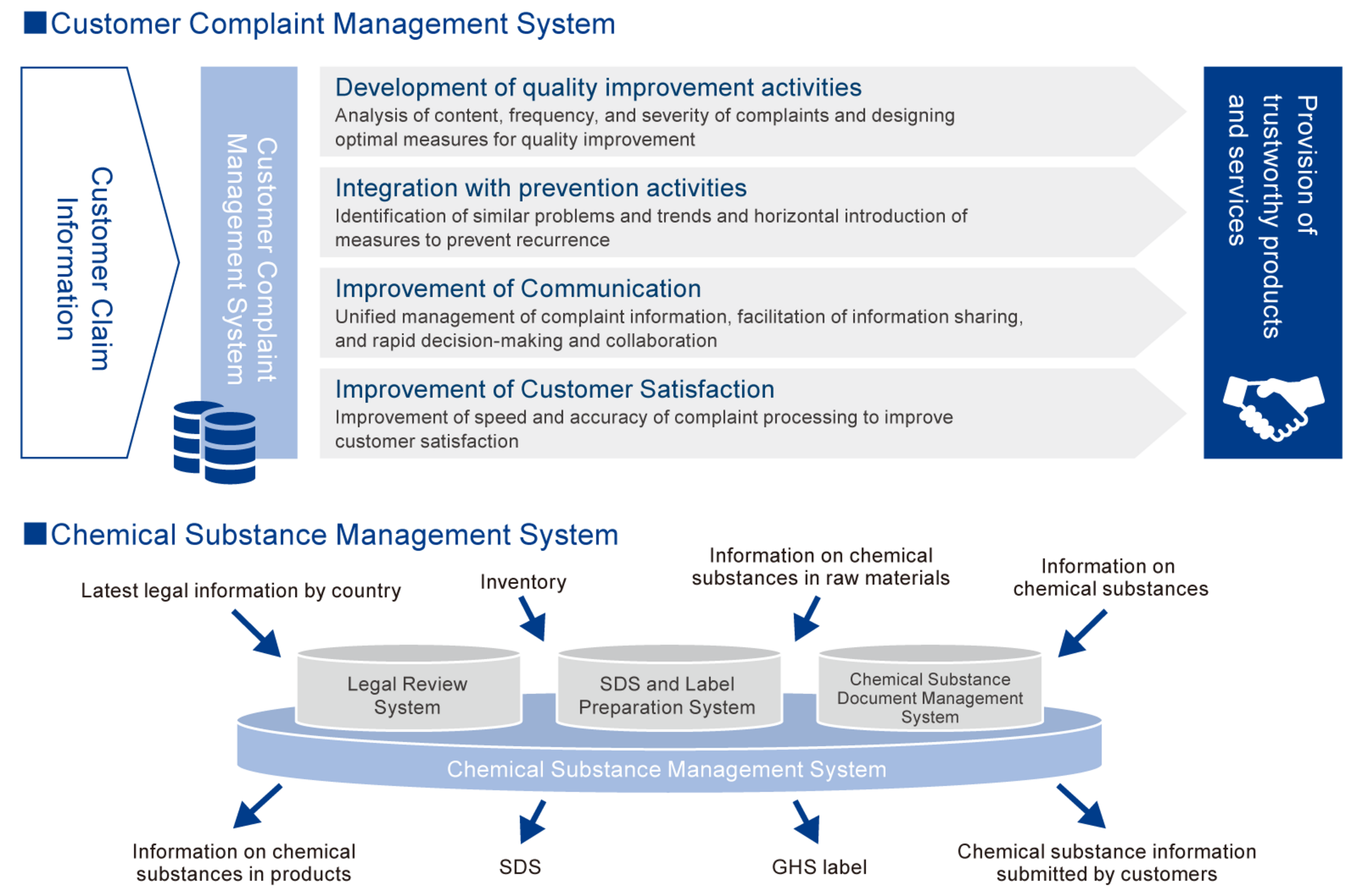
Our Group constantly monitors domestic and overseas legal revision information regarding chemical substances. For the information of chemical substances contained in products subject to legal revision, we provide revised SDSs and notify customers of the information on the contents of the products. If changes to product components or production processes are required to comply with revised laws and regulations, we will respond swiftly and appropriately.
The Denka Group has established the following Quality Assurance Policy, which is the highest internal regulation regarding quality assurance: "In regard to products, ensure compliance with laws and regulations and improve customer satisfaction." In compliance with this policy, the Denka Group conducts manufacturing and provision of reliable products under management in accordance with GMP Ordinance (Standards for Manufacturing Control and Quality Control for Pharmaceuticals)/GQP Ordinance (Standards for Quality Control for Pharmaceuticals)/GVP Ordinance (Standards for Post-Marketing Safety Management for Pharmaceuticals and Medical Devices)/QMS Ordinance (Standards for Manufacturing Control and Quality Control for Medical Devices and In Vitro Diagnostic Reagents) in the pharmaceutical business.
Furthermore, the Denka Group is a member of the Japan Vaccine Industry Association, the Japan Association of Clinical Reagents, and the Japan Medical Device Industry Association, and is working together with these organizations to contribute to the improvement of health and welfare for all people.
To provide reliable products and services, we are promoting the establishment of quality control systems.
By using systems such as the Complaint Management System, which integrates and manages customer complaint information to facilitate ongoing quality improvement, and the Chemical Substance Management System, which links information on chemical substances to product safety, we are raising our level of management and striving to further improve quality and ensure product safety.
In order to reflect customer feedback in our quality assurance activities, the Electronics and Innovative Products Division conducts a customer satisfaction survey once a year.
We analyze customer feedback from the aspects of sales, technology, quality, and delivery, and implement strategic actions for quality improvement.
Through customer satisfaction surveys, we aim to gain further trust from our stakeholders.
Our Company has established the Core Values of "Integrity" and "Innovation" as the foundation of our corporate activities. Based on these Core Values, we will continue to respond to the expectations and trust of many stakeholders, including shareholders, customers, local communities, and employees.
Our basic policy on BCP is as follows. Even in the event of a crisis or disaster that has a significant impact on our business activities, we will prioritize the safety of human life...
Our Philosophy and StandardsThe Denka Group has established materiality (important management issues) that it should address to achieve the SDGs. One of these is the...
Policy on the operation of the Board of Directors and roles and responsibilities Number of meetings held and attendance of each committee Implementation evaluation of the Board of Directors ...
List of DirectorsRepresentative Director, ChairmanToshio Imai(April 1982...
The Denka Group understands that compliance goes beyond the observance of laws and regulations, the Articles of Incorporation, and internal rules and regulations. It also includes the observance of corporate ethics and social norms as a good corporate citizen.
The Denka Group Code of Ethics establishes standards of conduct that must be followed by officers and employees of Denka Group companies in order to maximize corporate value. Denka is...
Established in October 2019, revised on July 1, 2023 Denka has established the Denka Group Ethics Regulations to ensure that all officers and employees of the Denka Group act in accordance with these Regulations.
In the "Legal Hazard Map" prepared by Denka, approximately 10 legal fields related to Denka Group businesses are selected to identify compliance risks across the Group.
The Legal Department conducts compliance training based on the Business Conduct Guidelines, which prohibit all forms of corruption, and the Legal Hazard Map.
Denka has established the Information Security Group Policy in accordance with ISO27001, an international standard for information security.
Denka's Risk Management System Integrated risk management and Risk Management Committee ...
1. Introduction 2. Persons Targeted and Contents 3. How to Make Reports 4. Response After Receipt of Reports 5. Protection of Whistleblowers
In order for Denka Group companies to conduct fair transactions with customers and suppliers, it is essential to examine the terms of contracts from legal and business perspectives...
Measures to Prevent CorruptionThe Denka Group prohibits all forms of corruption, including acts that violate laws and regulations such as bribery and excessive entertainment and gifts that go beyond common social practices...
Denka prohibits the export of products and technologies that may lead to the development and manufacture of weapons of mass destruction in accordance with the security export control established by the Denka Group Ethical Standards and Denka's company-wide common...
Denka, in order to maintain the fairness of securities transactions and the trust in securities markets, as well as to prevent the illicit use of the Company’s information assets, has established the Denka Group Ethical Guidelines and...
The Denka Group has established the Denka Group Ethical Regulations as the foundation of its compliance system to set forth standards of conduct for the entire Denka Group. In addition, the Denka Group has put in place the Denka Group Code of Conduct and the Denka Group CSR Procurement Guidelines to further promote compliance.
Strengthening of group ICT governance Information security basic policy Management of information posted on website Information se...
[Basic Policy] Main Intellectual Property Activities Intellectual Property Activities [Basic Policy]...
Basic Policy (Quality Policy) Quality assurance system and measures to prevent recurrence of quality misconduct Product safety and quality risk assessment...
In the Electronics and Innovative Products Division, we conduct a customer satisfaction survey once a year to accurately respond to customer requests and reflect the evaluations received in our quality assurance activities...